The short wavelength limit of the Lyman, Paschen and Balmer series, in the hydrogen spectrum, are denoted by λ L, λ P and λ B respectively. Arrange these wavelengths in increasing order. Lyman series transitions. A continuum of light passing through this gas will consequently result in Balmer series absorption. A continuum of light passing through this gas will consequently result in Paschen series absorption. Paschen series transitions.
An electron in the ground state can absorb energy and enter a higher energy level (excited state).
Lyman Series Vs Balmer Series
For hydrogen, an electron in the ground state occupies the first energy level (n=1)
For hydrogen, an electron in the excited state occupies an energy level greater than n=1 (ie, n=2, n=3 etc)
An excited electron will fall down to a lower energy level, emitting a photon of particular energy (and hence of a particular wavelength(1)) in the process.The greater the energy of the photon emitted, the shorter its wavelength is.
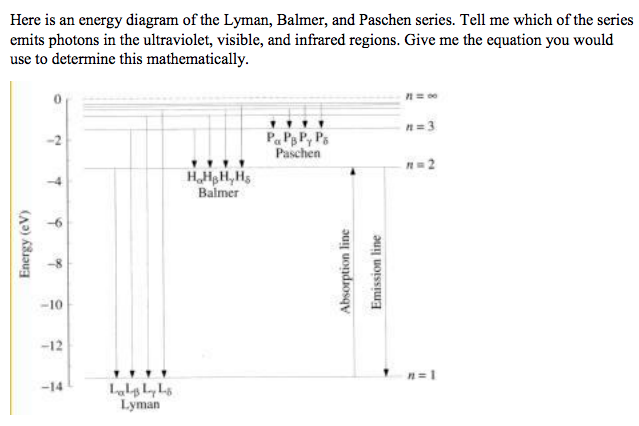
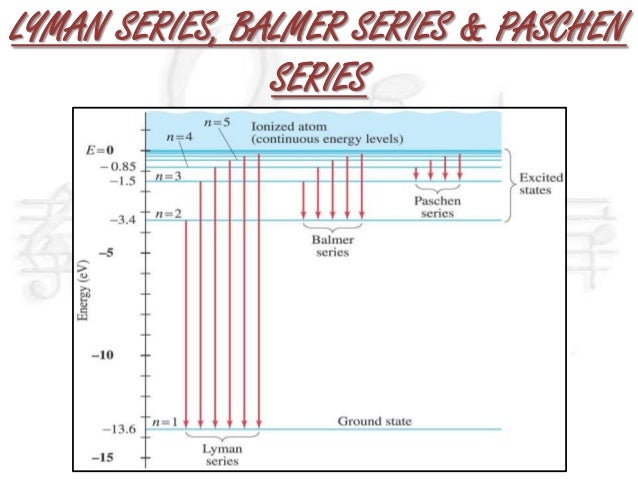
⚛ Lyman series : a group of lines in the ultraviolet region of the electromagnetic spectrum.
Lyman Balmer Paschen Brackett Pfund Series
⚛ Balmer series : a group of lines around the visible region of the electromagnetic spectrum.
Lyman Balmer Paschen Brackett Series
⚛ Paschen series : a group of lines in the infrared region of the electromagnetic spectrum.
⚛ Brackett series, Pfund series and Humphreys series also occur in the infrared region of the electromagnetic spectrum.
Each of these series corresponds to excited electrons falling down to a particular energy level:(2)
⚛ Lyman series : excited electrons fall back to the n=1 energy level
⚛ Balmer series : excited electrons fall back to the n=2 energy level
⚛ Paschen series : excited electrons fall back to the n=3 energy level
⚛ Brackett series : excited electrons fall back to the n=4 energy level
What Is The Difference Between Lyman And Balmer Series
⚛ Pfund series : excited electrons fall back to the n=5 energy level
Lyman Balmer Paschen Brackett Pfund
⚛ Humphreys series : excited electrons fall back to the n=6 energy level
