Copper, isotope of mass 66. Molecular Weight. 65.928869 g/mol. 561 copper isotopes products are offered for sale by suppliers on Alibaba.com, of which copper powder accounts for 18%, copper bars accounts for 1%. A wide variety of copper isotopes options are available to you, such as non-alloy, is alloy. There are 176 suppliers who sells copper isotopes on.
Atomic Weight
The atomic weight is the mass of an atom, typically expressed in atomic mass units (amu). For an isotope, it is the mass of the nucleus, that is the mass of the protons and neutrons, as the mass of the electrons are considered negligible. In their natural state only 21 elements exist as single isotopes, that is a sample has nuclei of only one isotope, and these are called the mononuclidic elements. Most elements exist as a mixture of nuclei from multiple isotopes, and these are labeled as the polynuclidic elements. The atomic weight of a monuclidic element is that mass of that nuclide.
Copper Isotopes Pie Graph
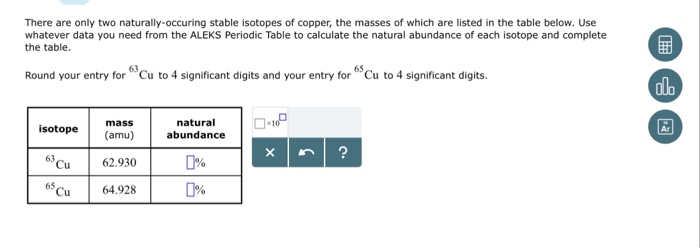
For a polynuclidic element the atomic weight is the average weight based on the fractional abundance of each isotope, and this is the value given on the periodic table. Copper has two isotopes, 63Cu (69.15%, mass=62.9300 amu) and 65Cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic weight of 63.55 amu, even though there is not a single atom that weighs 63.55 amu.
| [underbrace{0.6915}_{fraction ; ^{63}Cu}underbrace{(62.9300, amu)}_{mass ; ^{63}Cu} + underbrace{ 0.3085}_{fraction ; ^{65}Cu} underbrace{(64.928 ,amu)}_{mass ; ^{65}Cu} = underbrace{63.55, amu}_{text{average mass}} note: ; 0.6915 + 0.3085 = 1] |
Figure (PageIndex{1}): Natural samples of copper contain two isotopes, and its atomic weight is to four significant digits is 63.55 amu, even though there is not a single atom of copper that weights 63.55 amu.
Copper Isotopes
Exercise (PageIndex{1})
The atomic weight of chorine is ______________and the atomic number of chlorine-35 is________________.
Copper Isotopes Value
- 35, 17
- 17, 35
- 35.4527; 17
- 35.4527; 35

C) the atomic weight is the average of mass of all isotopes of chlorine atoms and found below the symbol on the periodic table. The atomic number is the number of protons in all chlorine atoms and is found on the top of the symbol in the periodic table.
Copper Isotopes Mass Spectrometry
Isotopes of the Element Copper
[Click for Main Data]

Most of the isotope data on this site has been obtained from the National Nuclear Data Center. Please visit their site for more information.
Isotopes With A Known Natural Abundance
| Mass Number | Natural Abundance | Half-life |
| 63 | 69.15% | STABLE |
| 65 | 30.85% | STABLE |
Known Isotopes
Copper Isotopes Buyers
| Mass Number | Half-life | Decay Mode | Branching Percentage |
| 52 | No Data Available | Proton Emission | No Data Available |
| 53 | < 300 nanoseconds | Electron Capture | No Data Available |
| Proton Emission | No Data Available | ||
| 54 | < 75 nanoseconds | Proton Emission | No Data Available |
| 55 | 27 milliseconds | Electron Capture | 100.00% |
| Electron Capture with delayed Proton Emission | 15.0% | ||
| 56 | 93 milliseconds | Electron Capture | 100.00% |
| Electron Capture with delayed Proton Emission | 0.40% | ||
| 57 | 196.3 milliseconds | Electron Capture | 100.00% |
| 58 | 3.204 seconds | Electron Capture | 100.00% |
| 59 | 81.5 seconds | Electron Capture | 100.00% |
| 60 | 23.7 minutes | Electron Capture | 100.00% |
| 61 | 3.333 hours | Electron Capture | 100.00% |
| 62 | 9.673 minutes | Electron Capture | 100.00% |
| 63 | STABLE | - | - |
| 64 | 12.701 hours | Electron Capture | 61.50% |
| Beta-minus Decay | 38.50% | ||
| 65 | STABLE | - | - |
| 66 | 5.120 minutes | Beta-minus Decay | 100.00% |
| 67 | 61.83 hours | Beta-minus Decay | 100.00% |
| 68 | 30.9 seconds | Beta-minus Decay | 100.00% |
| 68m | 3.75 minutes | Isomeric Transition | 84.00% |
| Beta-minus Decay | 16.00% | ||
| 69 | 2.85 minutes | Beta-minus Decay | 100.00% |
| 70 | 44.5 seconds | Beta-minus Decay | 100.00% |
| 70m | 33 seconds | Beta-minus Decay | 52.00% |
| Isomeric Transition | 48.00% | ||
| 70m1 | 6.6 seconds | Beta-minus Decay | 93.20% |
| Isomeric Transition | 6.80% | ||
| 71 | 19.4 seconds | Beta-minus Decay | 100.00% |
| 72 | 6.63 seconds | Beta-minus Decay | 100.00% |
| 73 | 4.2 seconds | Beta-minus Decay | 100.00% |
| 74 | 1.594 seconds | Beta-minus Decay | 100.00% |
| 75 | 1.222 seconds | Beta-minus Decay | 100.00% |
| Beta-minus Decay with delayed Neutron Emission | 3.50% | ||
| 76 | 637 milliseconds | Beta-minus Decay with delayed Neutron Emission | 7.20% |
| Beta-minus Decay | 100.00% | ||
| 76m | 1.27 seconds | Beta-minus Decay | 100.00% |
| 77 | 468.1 milliseconds | Beta-minus Decay | 100.00% |
| Beta-minus Decay with delayed Neutron Emission | 30.30% | ||
| 78 | 335 milliseconds | Beta-minus Decay | 100.00% |
| Beta-minus Decay with delayed Neutron Emission | > 65.00% | ||
| 79 | 188 milliseconds | Beta-minus Decay | 100.00% |
| Beta-minus Decay with delayed Neutron Emission | 55.00% | ||
| 80 | 0.17 seconds | Beta-minus Decay | No Data Available |
| 81 | > 632 nanoseconds | Beta-minus Decay with delayed Neutron Emission | No Data Available |
| Beta-minus Decay | No Data Available | ||
| Beta-minus Decay with delayed Double Neutron Emission | No Data Available | ||
| 82 | > 636 nanoseconds | Beta-minus Decay | No Data Available |
| Beta-minus Decay with delayed Neutron Emission | No Data Available | ||
| Beta-minus Decay with delayed Double Neutron Emission | No Data Available |
For questions about this page, please contact Steve Gagnon.
