››More information on molar mass and molecular weight. In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together.

What is the atomic number of carbon-14?
2 Answers
- The atomic mass is useful in chemistry when it is paired with the mole concept: the atomic mass of an element, measured in amu, is the same as the mass in grams of one mole of an element. Thus, since the atomic mass of iron is 55.847 amu, one mole of iron atoms would weigh 55.847 grams.
- ››More information on molar mass and molecular weight. In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all.
- Atomic Mass of Carbon Atomic mass of Carbon is 12.0107 u.
Explanation:

The atomic number is the number of protons, massive, positively charged nuclear particles, present in the nucleus of an atom.
Top bittorrent clients. Of course we gots the ISOTOPE,
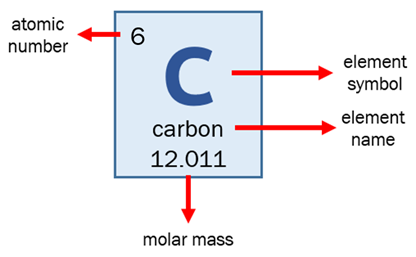
Explanation:


Atomic Mass Of Carbon 6
The bottom number of the atom is the atomic number which is based on the number of protons.
The 14 is the total mass of the atom. Latest version of mac os mojave. Carbon 14 has 6 protons and 8 neutrons for a total mass of 14
Atomic Mass Of Carbon 13
Related questions
