Lithium: isolation
Isolation: lithium would not normally be made in the laboratory as it is so readily available commercially. All syntheses require an electrolytic step as it is so difficult to add an electron to the poorly electronegative lithium ion Li+.
- Atomic Mass of Gold. Atomic mass of Gold is 196.9665 u. Note that, each element may contain more isotopes, therefore this resulting atomic mass is calculated from naturally-occuring isotopes and their abundance. The unit of measure for mass is the atomic mass unit (amu). One atomic mass unit is equal to 1.66 x 10-24 grams.
- How has the Model of the Atom Changed Over the Years? The Atomic Structure The historical development of atomic models: Over the last 100 years, scientists have done investigations which show that atoms are made up of even smaller particles. The atom was imagined as a small indivisible ball similar to a very tiny ball.
The ore spodumene, LiAl(SiO3)2, is the most important commercial ore containing lithium. Macintosh operating system download. The α form is first converted into the softer β form by heating to around 1100°C. This is mixed carefully with hot sulphuric acid and extracted into water to form lithium sulphate, Li2SO4, solution. The sulphate is washed with sodium carbonate, Na2CO3, to form a precipitate of the relatively insoluble lithium carbonate, Li2CO3.
Li2SO4 + Na2CO3 → Na2SO4 + Li2CO3 (solid)
Reaction of lithium carbonate with HCl then provides lithium chloride, LiCl.
Encryption consists in substituting letters (or group of letters) representing a chemical symbol by its corresponding atomic number. (Hydrogen H = 1, Helium He = 2, etc.) Example: FUSION can be decomposed in F 9 (Fluor), U 92 (Uranium), Si 14 (Silicium), O 8 (Oxygen), N 7 Nitrogen, so 9,92,14,8,7.
Li2CO3 + 2HCl → 2LiCl + CO2 +H2O
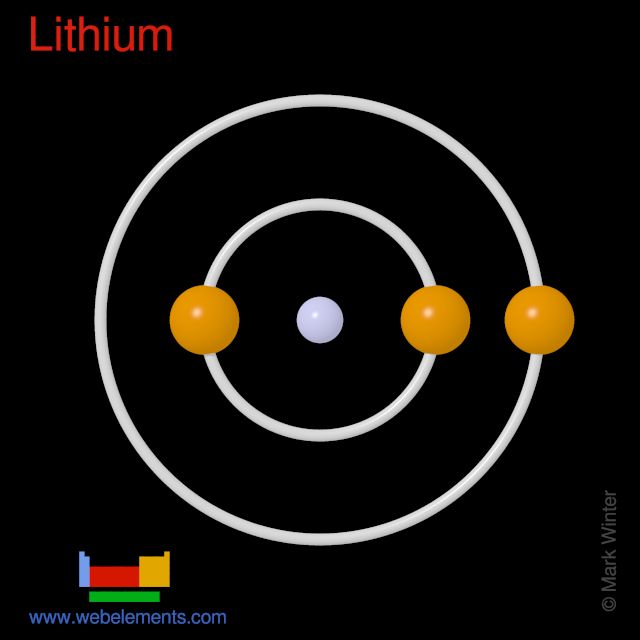
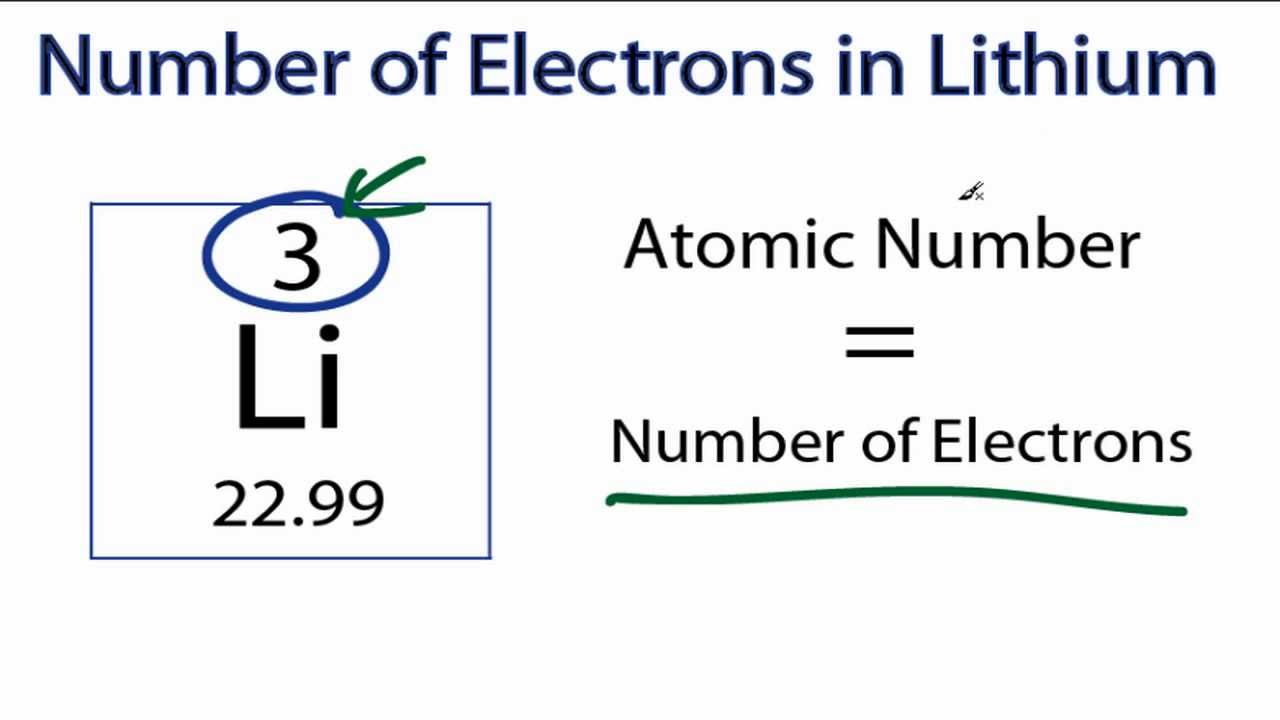
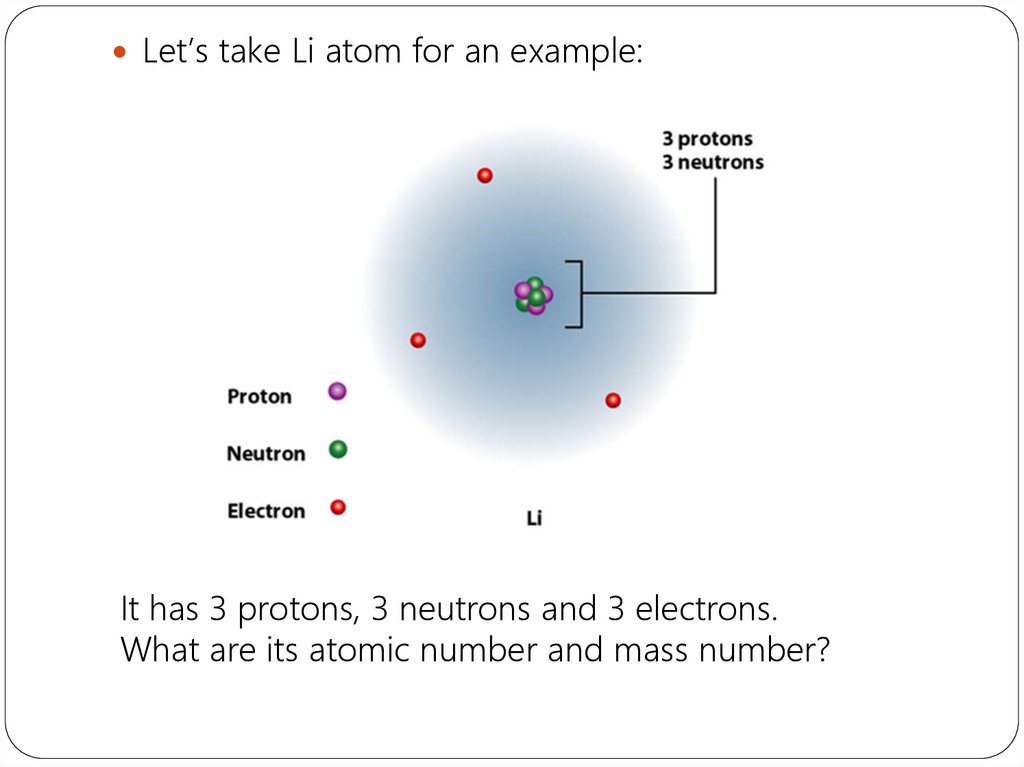
Li+1 Atomic Number
Lithium chloride has a high melting point (> 600°C) meaning that it sould be expensive to melt it in order to carry out the electrolysis. However a mixture of LiCl (55%) and KCl (45%) melts at about 430°C and so much less energy and so expense is required for the electrolysis.
Li-8 Atomic Number
Element Li Atomic Number
cathode: Li+(l) + e- → Li (l)
Li Atomic Mass Number
anode: Cl-(l) → 1/2Cl2 (g) + e-
